Inspections might in some cases be performed with other MHRA inspections, these types of just like good clinical practice or good pharmacovigilance practice.
No components really should be unveiled or employed prior to the satisfactory completion of analysis by the standard unit(s) Except there are actually suitable devices set up to allow for these types of use (e.
Normal good quality-testimonials of APIs should be executed with the target of verifying the regularity of the procedure. This sort of opinions really should Typically be carried out and documented annually and may involve a minimum of:
These information or copies thereof shall be topic to photocopying or other usually means of replica as Section of these types of inspection. Information that can be immediately retrieved from An additional area by Pc or other Digital signifies shall be regarded as Assembly the requirements of the paragraph.
Guidelines and treatments should be published in distinct and unambiguous language employing good documentation practices.
(a) Any manufacturing, Handle, or distribution document that is required for being taken care of in compliance using this portion and it is specifically connected to a batch of the drug merchandise shall be retained for at least 1 calendar year once the expiration date from the batch or, in the situation of certain OTC drug solutions lacking expiration relationship mainly because they meet the factors for exemption beneath § 211.137, 3 yrs following distribution from the batch.
GoAudits permits you to digitize SOPs, creating them very easily obtainable to the group anytime, any where. You may conduct frequent audits using customizable checklists that replicate your unique SOPs, making sure that every crew member follows the exact same protocols persistently.
To begin the Regulatory Compliance Associates scoping procedure now, be sure to enter your details within the blue variety underneath and click the submit button at the bottom in the click here webpage.
Not For Medical Use
Enhanced Performance and value Discounts: GMP will help in determining and correcting inefficiencies in the production process, which can lead to Charge personal savings. By optimizing operations and minimizing waste, providers can accomplish greater resource management and lessen generation charges.
(two) Every ingredient shall be analyzed for conformity with all correct prepared requirements for purity, energy, and excellent. In lieu of this sort of screening through the manufacturer, a report of analysis could possibly be accepted in the supplier of a component, provided that at the very least 1 specific identification exam is performed on this sort of element by the company, and furnished the company establishes the reliability of the provider's analyses by way of suitable validation of the provider's check effects at suitable intervals.
(h) Pending thought of a proposed exemption, posted while in the Federal Sign-up of September 29, 1978, the requirements Within this section shall not be enforced check here for human OTC drug merchandise if their labeling won't bear dosage limits and they are steady for at least three several years as supported by ideal balance info.
For GDP inspections your chance score is predicated on what things to do take place on web-site plus the quantity and sort of deficiencies observed. This means the probably day of your respective following inspection and this data is provided over the inspection report.
Records of manufacture (such as distribution) that allow the complete history of a batch to be traced must be retained in a comprehensible and accessible type.
 Michael Bower Then & Now!
Michael Bower Then & Now!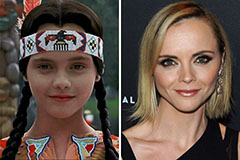 Christina Ricci Then & Now!
Christina Ricci Then & Now!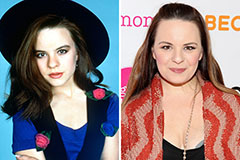 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Mackenzie Rosman Then & Now!
Mackenzie Rosman Then & Now! Jaclyn Smith Then & Now!
Jaclyn Smith Then & Now!